Scientists around the world use the metric
system. Even though it is not
the “official” system of the United States, scientists here use it too. There are many reasons for this. It provides a common language to communicate
findings; it is a decimal system that makes measuring easy; it is simple to
convert from one unit to another; and it is based on logical standards which
transcend measurements of lines, volume, mass, and temperature. In short, it’s a great system - which is why
the rest of the world adopted it long ago.
Measurement: Basic Unit: Tool: Original Definition:
Linear (length) meter (m) ruler 1/10000000 from N. Pole to
Equator
Volume (fluids) liter (l) graduate 1
cubic decimeter; also 1 kg of water
Mass gram (g) balance 1 cubic centimeter of water
Temperature degrees Celsius (°C) thermometer 1/100
from freezing to boiling water
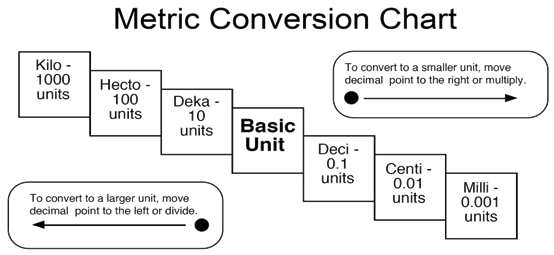
Prefix: Value: Name: Notation: Examples:
kilo- 1000 thousand 1.0 x 10 3 1
kilometer
= 1,000 meters
hecto- 100 hundred 1.0 x 10 2 100 liters = 1 hectoliter
deka- 10 ten 1.0 x 10 1 1 dekagram = 100 decigrams
(unit) 1 one 1.0 x 10 0
(meter, liter, gram)
deci- .1 tenth 1.0 x 10 -1 10 decigrams = 1 gram
centi- .01 hundredth 1.0 x 10 -2 0.01 meter = 1 centimeter
milli- .001 thousandth 1.0 x 10 -3 1 milliliter = 0.001 liters
Memory Aid: King
Henry Died until Drinking Chocolate Milk (K H D
u D C M)
Connections: 1
gram (g) = 1 milliliter (ml) = 1 cubic centimeter (cm3) of
water at 1 degree Celsius (0C)
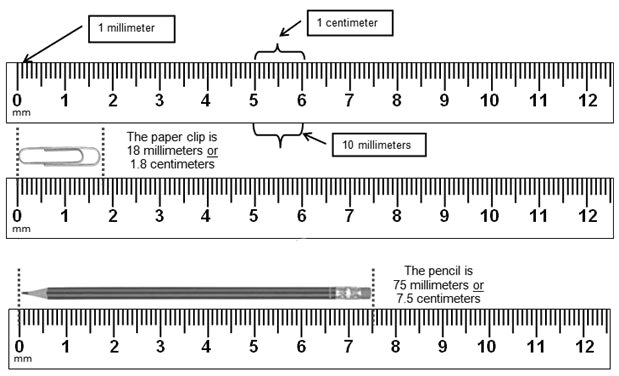
Linear
Measurements: basic unit = meter (m)
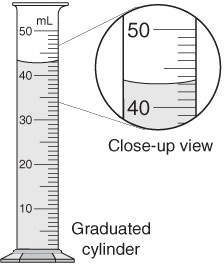
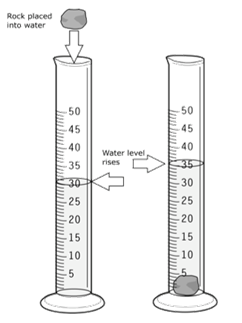
Volume
Measurements: basic unit = liter (L); (note: liquids = ml, solids = cm3)
Mass Measurements: basic
unit = gram (g)
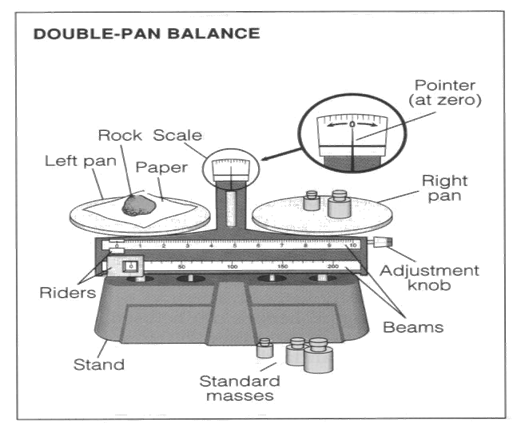

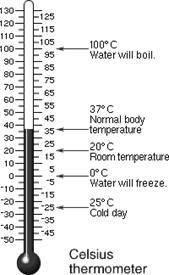
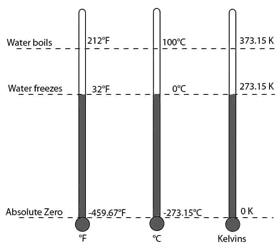
Temperature
Measurements: basic unit = degrees Celsius (0C)
 Graphing:
Graphing:
Formulas:
|
Density: |
density = mass /
volume |
D
= m / v |
|
Volume of Solid: |
volume = length x
width x height |
V
= (l)(w)(h) |
|
Speed: |
speed = distance /
time |
S
= d / t |
|
Acceleration: |
acceleration =
velocity change / time |
A
= (Vf – Vo) / t |
|
Momentum: |
momentum = mass x
velocity |
M
= (m)(v) |
|
Force: |
force = mass x
acceleration |
F
= (m)(a) |
|
Weight: |
weight = mass x
gravity |
w
= (m)(g) |
|
Pressure: |
pressure = force /
area |
P
= F/A |
|
Work: |
work = force x
distance |
W
= (F)(d) |
|
Power: |
power = work / time |
P
= W / t |
|
Kinetic Energy: |
kinetic energy =
(mass x velocity2)/2 |
KE
= (mv2) / 2 |
|
Potential Energy: |
potential energy = weight
x height |
PE
= (w)(h) |
|
Heat Gained / Lost: |
heat = mass x
Δ temp x specific heat |
H
= (m)(ΔT)(sp.ht.) |
|
Ohm’s Law: |
current = voltage /
resistance |
C
= V / R |
|
Electrical Power: |
power = voltage x
current |
P
= V / C |
|
Electrical Energy: |
energy = power x
time |
E
= (P)(t) |
|
Wave Speed: |
speed = frequency x
wavelength |
S
= (f)(λ) |
|
Law of Reflection: |
angle of incidence
= angle of reflection |
∠ i = ∠ r |
Significant
Digits:
